Cyclic dimeric adenosine 3′,5′-monophosphate (c-di-AMP) acts as a global regulator for various prokaryotic physiological processes and serves as a conserved microbial signature for innate immune detection of several bacterial pathogens. A recent study by Tang et al. (2022) discovered that bacterial stress caused by antibiotics increases c-di-AMP, which directly stimulates host inflammation by activating the Stimulator of Interferon Genes (STING). The Tang Lab is interested in (i) characterizing the precise mechanism by which antibiotic treatment modulates the host immune response through c-di-AMP and (ii) elucidating the role of c-di-AMP in regulating the viability and pathogenesis of Listeria monocytogenes under antibiotic stress.
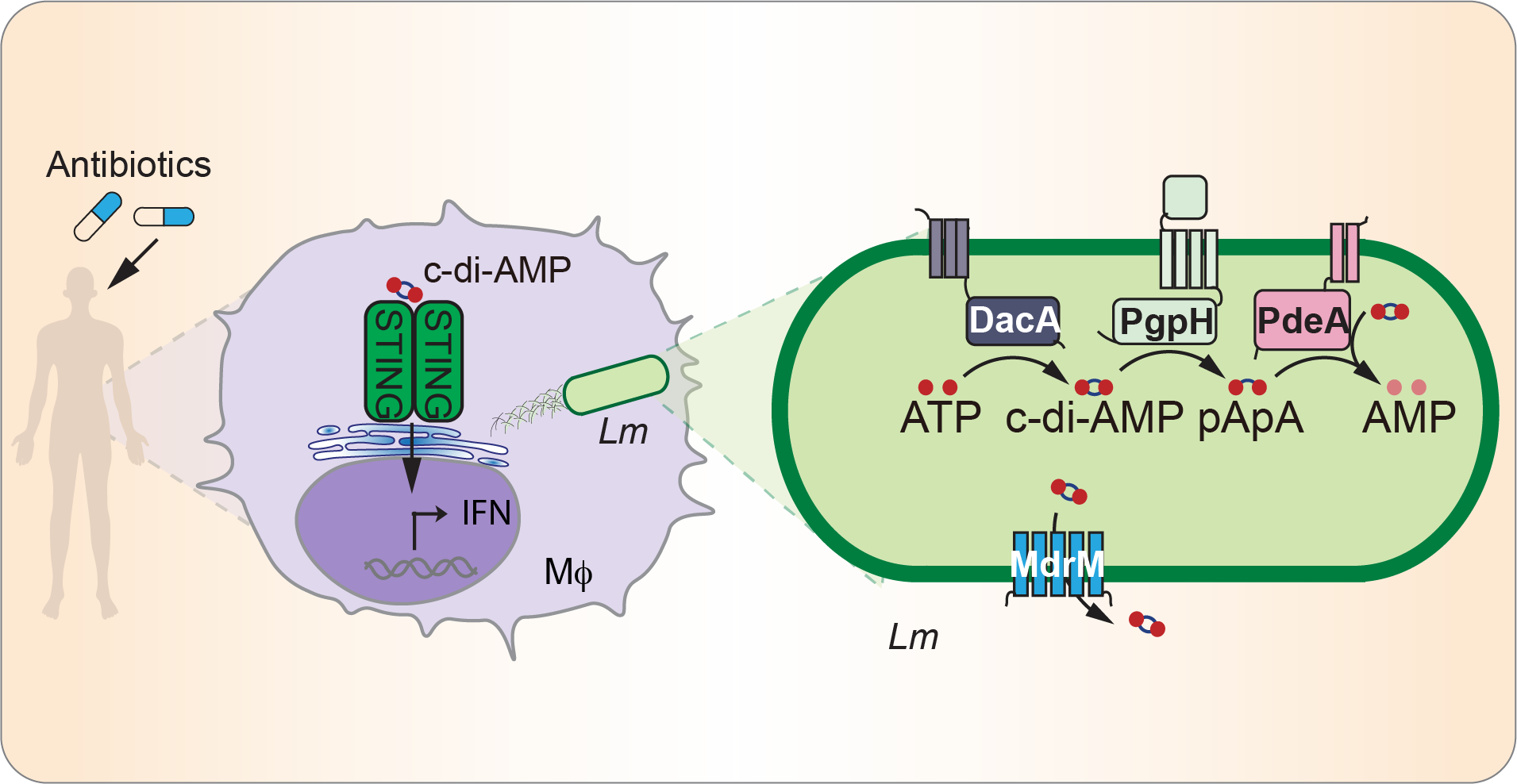
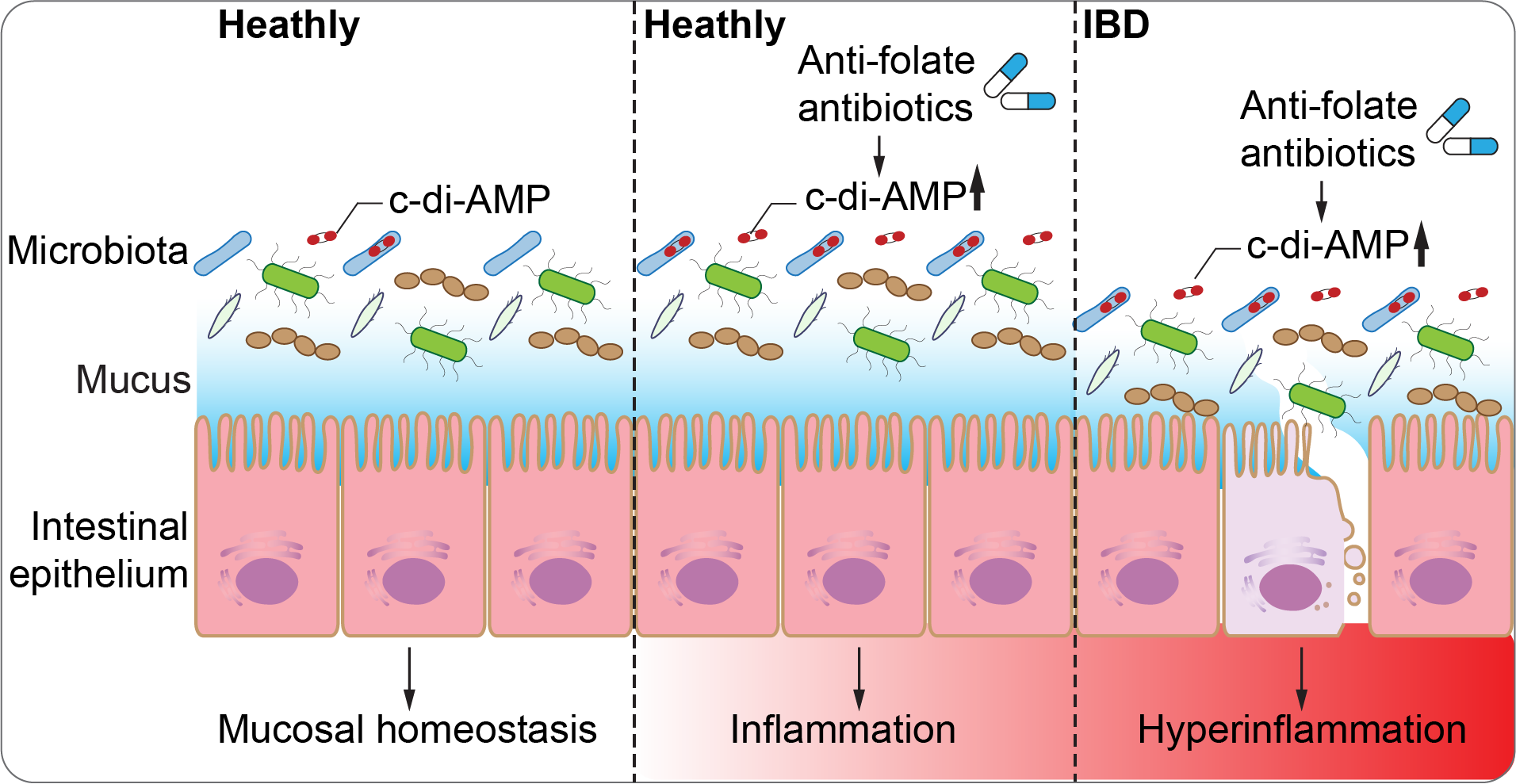
Numerous studies have demonstrated that antibiotic treatment decreases the diversity of microbiota species, potentially leading to gastrointestinal inflammation or susceptibility to pathogen infections. However, the effects of antibiotics on microbiota physiology and host immune homeostasis remain largely unexplored. The Tang lab is dedicated to investigating how antibiotics impact intestinal immune homeostasis and the development of inflammatory bowel disease by altering the microbiota’s physiological processes. Specifically, we are focusing on the role of c-di-AMP, produced by dominant phyla in the human gut microbiota such as Firmicutes, Bacteroidetes, and Actinobacteria, which activates the STING pathway. The Tang lab is actively characterizing this novel aspect of antibiotic treatment and its influence on mucosal immunity through c-di-AMP signaling.
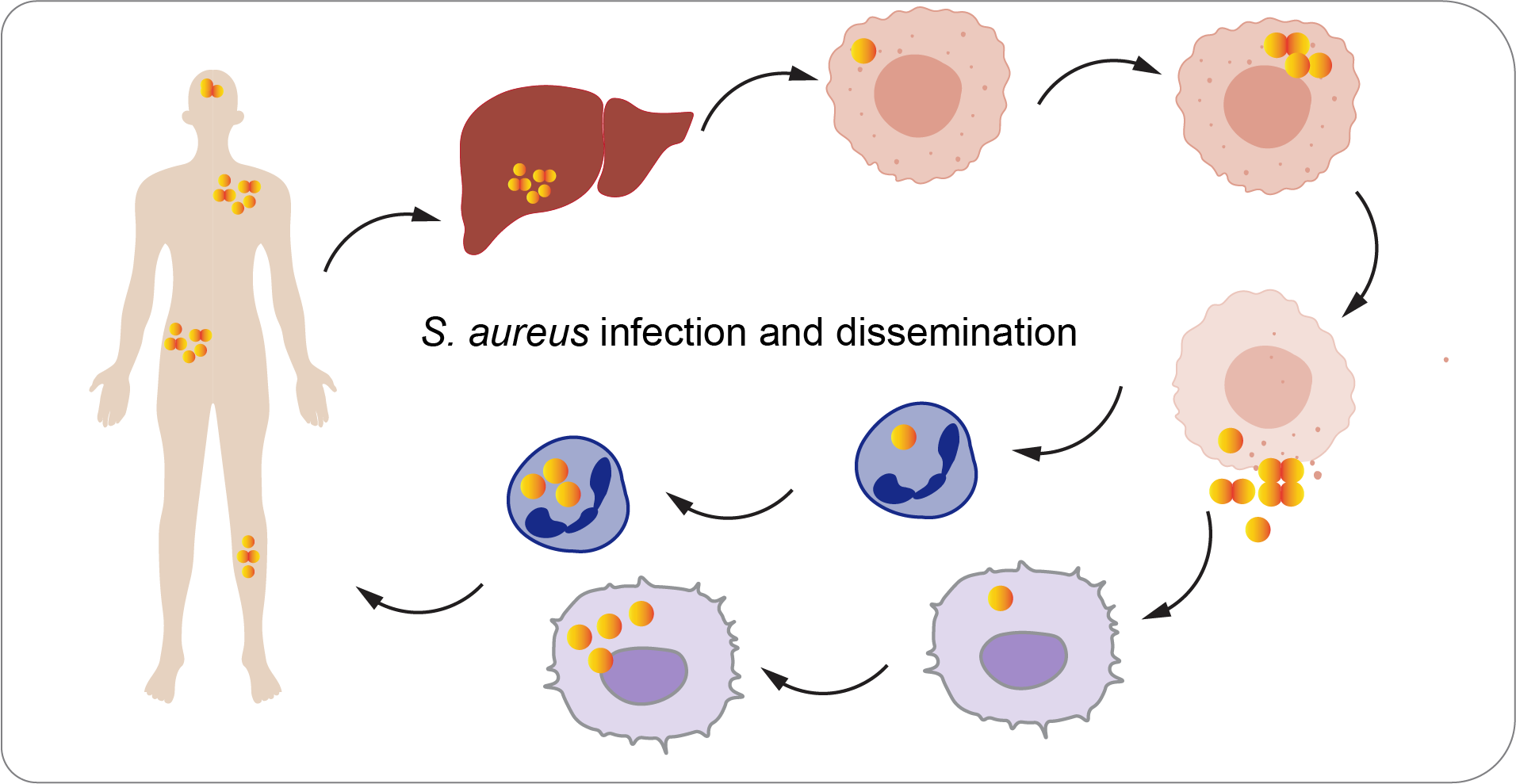
Methicillin-Resistant Staphylococcus aureus (MRSA) infections result in significant mortality around the world. The Tang Lab focuses on investigating the pathogenesis of S. aureus through murine lung infection and systemic infection models. Our research involves utilizing Tn-seq to identify cell factors that are essential for the survival of S. aureus in both macrophages and mice.